Examples of SEM Coating Fluid on biological samples
Sputter targets
Conductive SEM Coating Fluid
Examples of SEM Coating Fluid on material samples
Examples of SEM Coating Fluid on biological samples
back to BEL-1 conductive ionic SEM coating fluid page
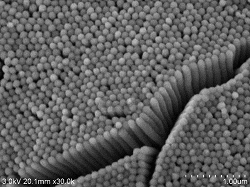
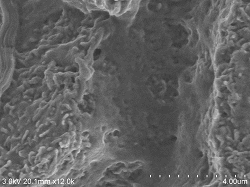
Renal glomerular surface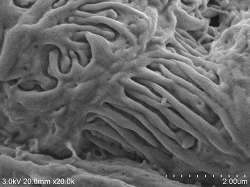
Sample preparation conditions (4 ° C): After fixation with 2% glutaraldehyde perfusion, chopped (thickness approx. 0.5 mm) and re-fixed ⇒ 1% osmium tetroxide fixed 2 hours ⇒ DW wash 10 minutes, 6 times ⇒ 1% tannin Acid aqueous solution 3 hours ⇒ DW washing 10 minutes, 6 times ⇒ 1% osmium tetroxide fixation 2 hours ⇒ DW washing 10 minutes, 3 times ⇒ 2% BEL-1 (70% ethanol) 1.5 hour immersion ⇒ Blower, cold air dryer drying Observation Conditions: SE detector (mixed mix), acceleration voltage 3KV, WD20, emission current (0.8 to 4.7μA) |
Rat kidney kidney glomerulus Sample preparation conditions (4 ° C): After fixation with 2% glutaraldehyde perfusion, chopped (thickness approx. 0.5 mm) and re-fixed ⇒ 1% osmium tetroxide fixed 2 hours ⇒ DW wash 10 minutes, 6 times ⇒ 1% tannin Acid aqueous solution 3 hours ⇒ DW washing 10 minutes, 6 times ⇒ 1% osmium tetroxide fixation 2 hours ⇒ DW washing 10 minutes, 3 times ⇒ 2% BEL-1 (70% ethanol) 1.5 hour immersion ⇒ Blower, cold air dryer drying Observation Conditions: SE detector (mixed mix), acceleration voltage 3KV, WD20, emission current (0.8 to 4.7μA) |
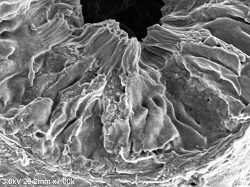
Rat kidney proximal tubule (brush border) Sample preparation conditions (4 ° C): After fixation with 2% glutaraldehyde perfusion, chopped (thickness approx. 0.5 mm) and re-fixed ⇒ 1% osmium tetroxide fixed 2 hours ⇒ DW wash 10 minutes, 6 times ⇒ 1% tannin Acid aqueous solution 3 hours ⇒ DW washing 10 minutes, 6 times ⇒ 1% osmium tetroxide fixation 2 hours ⇒ DW washing 10 minutes, 3 times ⇒ 2% BEL-1 (70% ethanol) 1.5 hour immersion ⇒ Blower, cold air dryer drying Observation Conditions: SE detector (mixed mix), acceleration voltage 3KV, WD20, emission current (0.8 to 4.7μA) |
Rat kidney rlomerular cleavage image (inner blood vessel) Sample preparation conditions (4 ° C): After fixation with 2% glutaraldehyde perfusion, chopped (thickness approx. 0.5 mm) and re-fixed ⇒ 1% osmium tetroxide fixed 2 hours ⇒ DW wash 10 minutes, 6 times ⇒ 1% tannin Acid aqueous solution 3 hours ⇒ DW washing 10 minutes, 6 times ⇒ 1% osmium tetroxide fixation 2 hours ⇒ DW washing 10 minutes, 3 times ⇒ 2% BEL-1 (70% ethanol) 1.5 hour immersion ⇒ Blower, cold air dryer drying Observation Conditions: SE detector (mixed mix), acceleration voltage 3KV, WD20, emission current (0.8 to 4.7μA) |
Rat kidney proximal tubule (brush border) Sample preparation conditions (4 ° C): After fixation with 2% glutaraldehyde perfusion, chopped (thickness approx. 0.5 mm) and re-fixed ⇒ 1% osmium tetroxide fixed 2 hours ⇒ DW wash 10 minutes, 6 times ⇒ 1% tannin Acid aqueous solution 3 hours ⇒ DW washing 10 minutes, 6 times ⇒ 1% osmium tetroxide fixation 2 hours ⇒ DW washing 10 minutes, 3 times ⇒ 2% BEL-1 (70% ethanol) 1.5 hour immersion ⇒ Blower, cold air dryer drying Observation Conditions: SE detector (mixed mix), acceleration voltage 3KV, WD20, emission current (0.8 to 4.7μA) |
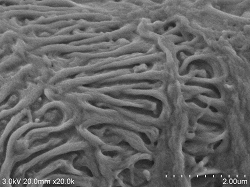
at liver capillary bile duct Sample preparation conditions (4 ° C): After fixation with 2% glutaraldehyde perfusion, chopped (thickness approx. 0.5 mm) and re-fixed ⇒ 1% osmium tetroxide fixed 2 hours ⇒ DW wash 10 minutes, 6 times ⇒ 1% tannin Acid aqueous solution 3 hours ⇒ DW washing 10 minutes, 6 times ⇒ 1% osmium tetroxide fixation 2 hours ⇒ DW washing 10 minutes, 3 times ⇒ 2% BEL-1 (70% ethanol) 1.5 hour immersion ⇒ Blower, cold air dryer drying Observation Conditions: SE detector (mixed mix), acceleration voltage 3KV, WD20, emission current (0.8 to 4.7μA) |
Rat liver rascular endothelial cells Sample preparation conditions (4 ° C): After fixation with 2% glutaraldehyde perfusion, chopped (thickness approx. 0.5 mm) and re-fixed ⇒ 1% osmium tetroxide fixed 2 hours ⇒ DW wash 10 minutes, 6 times ⇒ 1% tannin Acid aqueous solution 3 hours ⇒ DW washing 10 minutes, 6 times ⇒ 1% osmium tetroxide fixation 2 hours ⇒ DW washing 10 minutes, 3 times ⇒ 2% BEL-1 (70% ethanol) 1.5 hour immersion ⇒ Blower, cold air dryer drying Observation Conditions: SE detector (mixed mix), acceleration voltage 3KV, WD20, emission current (0.8 to 4.7μA) |
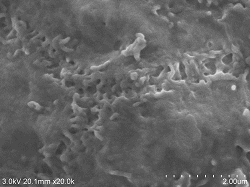
Rat small intestine Microvilli Sample preparation conditions (4 ° C): After fixing with 2% glutaraldehyde, shred (thickness approx. 0.5 mm) and re-fix ⇒ Fix with 1% osmium tetroxide 2 hours ⇒ DW wash 10 minutes, 6 times ⇒ 1% tannic acid Aqueous solution 3 hours ⇒ DW washing 10 minutes, 6 times ⇒ 1% osmium tetroxide fixation 2 hours ⇒ DW washing 10 minutes, 3 times ⇒ 2% BEL-1 (70% ethanol) 2 hours immersion ⇒ Blower, cold air dryer drying Observation conditions: SE detector (mixed mix), acceleration voltage 3KV, WD20, emission current (0.8 to 4.7μA) |
Rat kidney glomerular cleavage image (inner blood vessel)
Double fixing ⇒ 5% BEL-1 (70% ethanol) immersion for 2 hours ⇒ Drying (blower, cold air dryer) |
Proximal tubule (brush border) Double fixing ⇒ 5% BEL-1 (70% ethanol) immersion for 2 hours ⇒ Drying (blower, cold air dryer) |
Kidney glomerulus Double fixing ⇒ 5% BEL-1 (70% ethanol) immersion for 2 hours ⇒ Drying (blower, cold air dryer) |
Rat kidney proximal tubule (brush border) Double fixing ⇒ 5% BEL-1 (70% ethanol) immersion for 2 hours ⇒ Drying (blower, cold air dryer) |
Renal glomerular surface Double fixing ⇒ 5% BEL-1 (70% ethanol) immersion for 2 hours ⇒ Drying (blower, cold air dryer) |
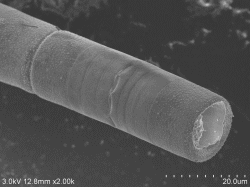
Rat small intestine soft projection Double fixing ⇒ 5% BEL-1 (70% ethanol) immersion for 2 hours ⇒ Drying (blower, cold air dryer) |
Rat liver capillary bile duct Double fixing ⇒ 5% BEL-1 (70% ethanol) immersion for 2 hours ⇒ Drying (blower, cold air dryer) |
Rat liver capillary bile duct Double fixing ⇒ 5% BEL-1 (70% ethanol) immersion for 2 hours ⇒ Drying (blower, cold air dryer) |
Neutrophil 1% GA fixation (slide glass with PLL coating) 30 minutes adhesion (wet) ⇒ DW cleaning ⇒ 10% BEL-1 (diluted solution: 100% ethanol) 10 minutes immersion ⇒ Drying (blower → cold air dryer about 15 minutes) Observation conditions : SE detector Upper emission current 5.5μA |
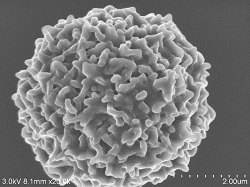
Diatoms 2% GA fixed ⇒ DW cleaning ⇒ 10% BEL-1 (70% ethanol) 10 minute immersion ⇒ Drying (blower, cold air dryer) Observation conditions: Acceleration voltage 3KV Emission current: 5.5μA SE detector: Mix |
Diatoms 1% GA ⇒ DW washing ⇒ 10% BEL-1 (70% ethanol) 10 minutes ⇒ Dry (cold air dryer) |
Diatoms 1% GA ⇒ DW washing ⇒ 10% BEL-1 (70% ethanol) 10 minutes ⇒ Dry (cold air dryer) |
Megalopa larva 1% GA ⇒ DW washing ⇒ 10% BEL-1 (70% ethanol) 10 minutes ⇒ Dry (cold air dryer) |
Megalopa larva 1% GA ⇒ DW washing ⇒ 10% BEL-1 (70% ethanol) 10 minutes ⇒ Dry (cold air dryer) |
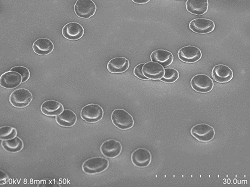
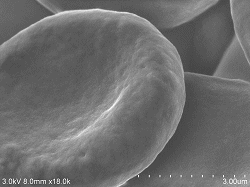
Red blood cells 10% (diluted solution: 100% ethanol) 10 minutes immersion Acceleration voltage: 3kV Emission current: 5.5μA SE detector: Upper |
White blood cells 10% (diluted solution: 100% ethanol) 10 minutes immersion Acceleration voltage: 3kV Emission current: 5.5μA SE detector: Upper |
SEM slide glass used 1% GA fixation (SEM slide glass) 30 minutes adhesion (wet) ⇒ DW cleaning ⇒ 10% BEL-1 (diluted solution: 70% ethanol) 10 minutes immersion ⇒ Drying (blower → cold air dryer about 15 minutes) Observation conditions: SE detector Upper emission current 5.5μA |
SEM slide glass used 1% GA fixation (SEM slide glass) 30 minutes adhesion (wet) ⇒ DW cleaning ⇒ 10% BEL-1 (diluted solution: 70% ethanol) 10 minutes immersion ⇒ Drying (blower → cold air dryer about 15 minutes) Observation conditions: SE detector Upper emission current 5.5μA |
SEM slide glass used 1% GA fixation (SEM slide glass) 30 minutes adhesion (wet) ⇒ DW cleaning ⇒ 10% BEL-1 (diluted solution: 70% ethanol) 10 minutes immersion ⇒ Drying (blower → cold air dryer about 15 minutes) Observation conditions: SE detector Upper emission current 5.5μA |
SEM slide glass used < <1% GA fixation (SEM slide glass) 30 minutes adhesion (wet) ⇒ DW cleaning ⇒ 10% BEL-1 (diluted solution: 70% ethanol) 10 minutes immersion ⇒ Drying (blower → cold air dryer about 15 minutes) Observation conditions: SE detector Upper emission current 5.5μA |
Courtesy of St. Marianna University School of Medicine, Electron Microscope Research Facility Chisako Sasaki
back to BEL-1 conductive ionic SEM coating fluid page